Vascular and Structural Heart Component Manufacturing
Implantable Heart Solutions Components:
Tricuspid, Mitral & Aortic Valves: When the human heart valve becomes severely diseased and can no longer function adequately to pump and prevent leakage of blood, it may become necessary to replace one or more of those valves with an artificial version.
Atrial Appendage Occlusion (LAAO) Devices: Atrial fibrillation can result in blood clots dislodging from the left atrial appendage, resulting stroke.
Aortic Valve Sewing Rings: When a heart valve is replaced with a surgical prosthetic, it must sit securely in place and be able to withstand the force of the beating heart as well as the passage of blood through the chambers.
Embolic Protection Devices:
This is a class of products that plays an important role in preventing stroke during procedures such as TAVR. An embolic protection device acts as a physical barrier, preventing particles and debris from traveling to the brain, lungs, and kidneys where they can occlude critical passageways for blood and oxygen.
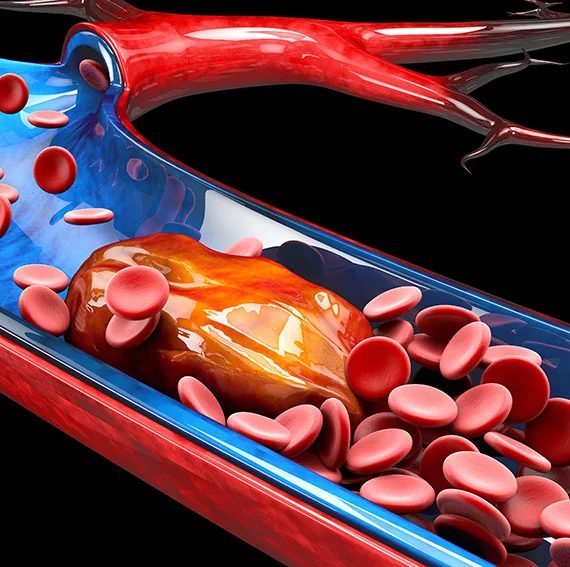
Surgical Grafts:
The Vascular graft can be made in a number of different configurations including straight, bifurcated, branched, and Valsalva. The grafts themselves are frequently made from knit or woven tubes. Woven grafts tend to have the strongest construction and are highly resistant to dilatation.
For more information about our custom implantable vascular and structural heart solutions, give ATEX’s engineering team a call today. Our innovative approach, coupled with extensive engineering and technical abilities, can be harnessed to create exactly the component you need for your medical device.
![]()
Let's partner and innovate together
Have questions? Ready to get started? ATEX
is available to discuss the scope of your
component needs.